Symbol Glossary Definitions
| SYMBOL | STANDARD REFERENCE | STANDARD TITLE | SYMBOL TITLE | EXPLANATORY TEXT |
|---|---|---|---|---|

|
ISO 15223-1:2021 Reference no. 5.1.1 |
Medical devices — Symbols to be used with information to be supplied by the manufacturer – Part 1: General requirements | Manufacturer | Indicates the medical device manufacturer. |

|
ISO 15223-1:2021 Reference no. 5.1.2 |
Medical devices — Symbols to be used with information to be supplied by the manufacturer – Part 1: General requirements | Authorized Representative in the European Community/ European Union | Indicates the authorized representative in the European Community/European Union. |

|
ISO 15223-1:2021 Reference no. 5.1.3 |
Medical devices — Symbols to be used with information to be supplied by the manufacturer – Part 1: General requirements | Date of manufacture | Indicates the date when the medical device was manufactured. |
|
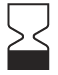
ISO 15223-1:2021 Reference no. 5.1.4 |
Medical devices — Symbols to be used with information to be supplied by the manufacturer – Part 1: General requirements | Use-by date | Indicates the date after which the medical device is not to be used. |

|
ISO 15223-1:2021 Reference no. 5.1.5 |
Medical devices — Symbols to be used with information to be supplied by the manufacturer – Part 1: General requirements | Batch code |
Indicates the manufacturer’s batch code so that the batch or lot can be identified. Synonyms for “batch code” are “lot number”, “lot code”, and “batch number”. |

|
ISO 15223-1:2021 Reference no. 5.1.6 |
Medical devices — Symbols to be used with information to be supplied by the manufacturer – Part 1: General requirements | Catalogue number or Catalog number | Indicates the manufacturer’s catalogue/catalog number so that the medical device can be identified. |

|
ISO 15223-1:2021 Reference no. 5.1.8 |
Medical devices — Symbols to be used with information to be supplied by the manufacturer – Part 1: General requirements | Importer | Indicates the entity importing the medical device into the locale. |

|
ISO 15223-1:2021 Reference no. 5.1.9 |
Medical devices — Symbols to be used with information to be supplied by the manufacturer – Part 1: General requirements | Distributor | Indicates the entity distributing the medical device into the locale. |
|
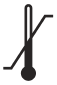
ISO 15223-1:2021 Reference no. 5.3.7 |
Medical devices — Symbols to be used with information to be supplied by the manufacturer – Part 1: General requirements | Temperature limit | Indicates the temperature limits to which the medical device can be safely exposed. |

|
ISO 15223-1:2021 Reference no. 5.4.1 |
Medical devices — Symbols to be used with information to be supplied by the manufacturer – Part 1: General requirements | Biological risks | Indicates that there are potential biological risks associated with the medical device. |

|
ISO 15223-1:2021 Reference no. 5.4.3 |
Medical devices — Symbols to be used with information to be supplied by the manufacturer – Part 1: General requirements | Consult instructions for use or consult electronic instructions for use | Indicates the need for the user to consult the instructions for use. |

|
ISO 15223-1:2021 Reference no. 5.5.1 |
Medical devices — Symbols to be used with information to be supplied by the manufacturer – Part 1: General requirements | In Vitro diagnostic medical device | Indicates a medical device intended to be used as an in vitro diagnostic medical device. |

|
N/A | N/A | Content | Indicates the content in the product. |

|
N/A | N/A | Reagent 1 | Indicates Reagent 1 in the product. |

|
N/A | N/A | Reagent 2 | Indicates Reagent 2 in the product. |

|
ISO 15223-1:2021 Reference no. 5.5.3 |
Medical devices — Symbols to be used with information to be supplied by the manufacturer – Part 1: General requirements | Negative control | Indicates a control material that is intended to verify the results in the expected negative range. |

|
ISO 15223-1:2021 Reference no. 5.5.4 |
Medical devices — Symbols to be used with information to be supplied by the manufacturer – Part 1: General requirements | Positive control | Indicates a control material that is intended to verify the results in the expected positive range. |

|
N/A | N/A | SDS | Indicates Safety Data Sheet. |

|
ISO 15223-1:2021 Reference no. 5.5.5 |
Medical devices — Symbols to be used with information to be supplied by the manufacturer – Part 1: General requirements | Contains sufficient for number of tests | Indicates the total number of tests that can be performed with the medical device. |

|
ISO15223-1:2021 Reference no. 5.7.10 |
Medical devices — Symbols to be used with information to be supplied by the manufacturer – Part 1: General requirements | Unique device identifier | Indicates a carrier that contains unique device identifier information. |

|
N/A | N/A | Global Trade Item Number | Indicates that the product has a Global Trade Item Number. |

|
Regulation (EU) 2017/746 Article 18 Annex V |
Regulation (EU) 2017/746 of the European Parliament and of the Council of 5th April 2017 on in vitro diagnostic medical devices and repealing Directive 98/79/EC and Commission Decision 2010/227/EU | CE marking | ‘CE marking of conformity’ or ‘CE marking’ means a marking by which a manufacturer indicates that a device is in conformity with the applicable requirements set out in this Regulation and other applicable Union harmonization legislation providing for its affixing. (IVDR Article 2, (35)). |

|
N/A | N/A | Prescription Use Only | Caution: Federal law (USA) restricts this device to sale by or on the order of a licensed healthcare practitioner. |
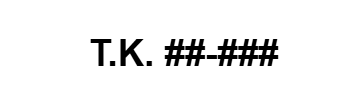
|
N/A | N/A | Test Kit Number | Canadian Registration for Test Kit. |
